Two proteins in the brain enrich the ingredients for gene translation every 24-hours, leading to rhythmic changes in the bodies of mice — and perhaps people.
The physiological and biological activity of many living things follows a roughly 24-hour cycle. This so-called circadian rhythm explains why we get sleepy at night and feel more alert in the morning.
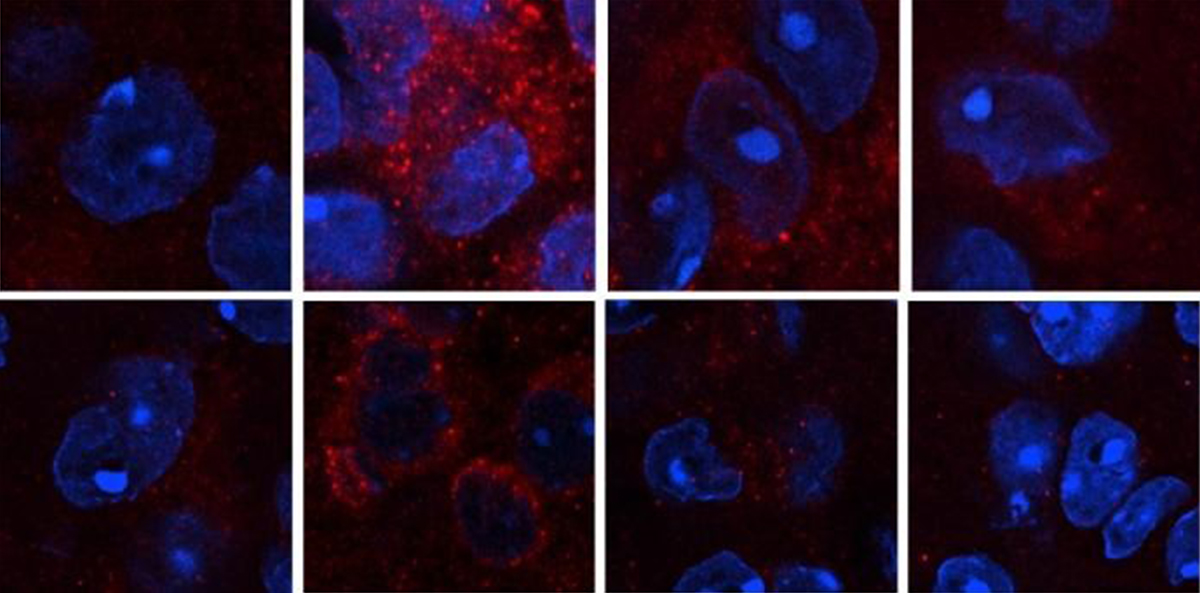
It turns out these structures in the brain assemble and disassemble following a daily pattern.
Now scientists have discovered special switches that may control the process: proteins that assemble to form ‘liquid-like condensates’ and then disassemble in the brain every 24 hours, following a rhythmic pattern.
It turns out that the condensation of these proteins plays an important role in controlling various rhythmic physiological processes in the body, including the switch between sleep and wakefulness, as well as the release of hormones, explains Yi Lin, the lead author of a new paper on the find, published in the journal Cell in July 2023.
While the study was carried out on mice, the results could help scientists understand the disruption of circadian rhythms in people with conditions such as diabetes, cancer and depression.
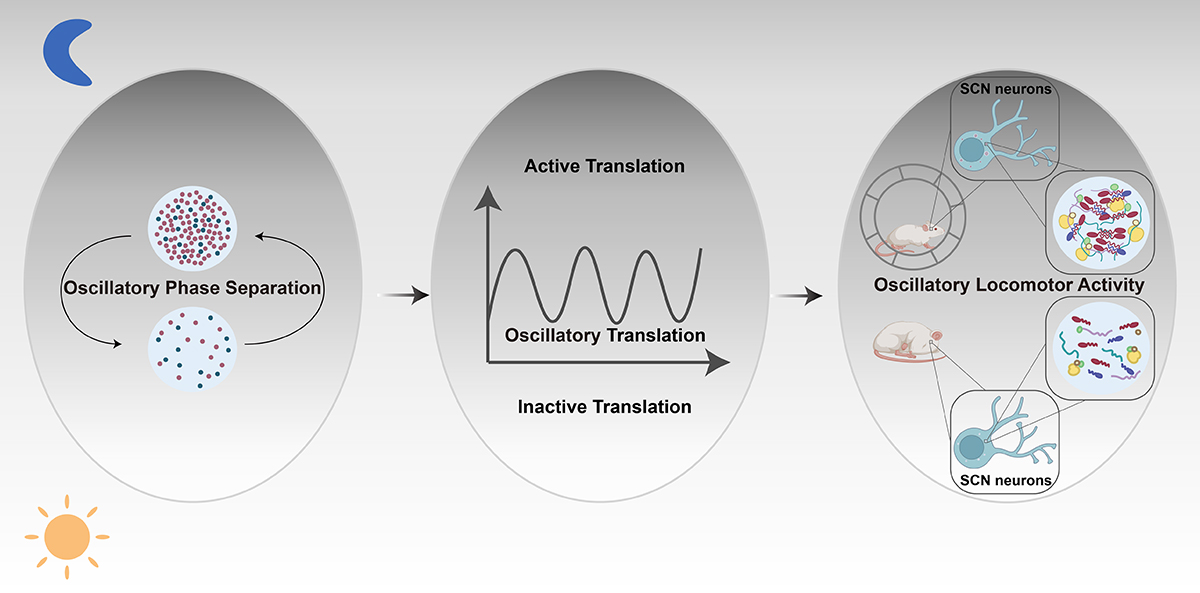
Liquid-like protein condensates in the brain assemble and disassemble following a rhythmic pattern that likely facilitates daily protein synthesis in the human body, among other physiological processes.
Timed translation
Researchers have long been fascinated by how the body’s circadian rhythm tracks the cycle of day and night. They have already unlocked some of the mechanisms, including transcription, translation and modification of the ‘clock genes’ that influence bodily activities such as the release of hormones.
However, a major question has been how these biological processes are controlled on a 24-hour schedule that, for example, makes levels of protein synthesis ebb and flow across a day.
To investigate, a Tsinghua University team looked at the action of proteins called Ataxin-2 and Ataxin-2L in the brain’s suprachiasmatic nucleus, a region of the brain’s hypothalamus that helps manage body temperature, hunger, thirst, mood, sex drive, blood pressure and desire to sleep.
They found that every 24 hours the Ataxin-2 protein formed condensates that locally enrich the ribosomes, translation initiation machinery, and specific RNAs, all of which cells need for gene translation — the process through which proteins are made.
Lin says that inside the suprachiasmatic nucleus, the regular enrichment of gene translation machinery by the Ataxin-2 protein boosts the expression of the clock genes Per2 and Cry1, on a 24-hour cycle.
Per2 and Cry1 are associated with processes such as sleep and the release of neurotransmitters, including dopamine. Changes to these genes have also been linked to sleep disorders, neurodegenerative disorders, drug addiction and depression, among other things.

Yi Lin (at center, front row) an Assistant Professor in Tsinghua University’s School of Life Science, and her team.
Treatment take-away
Lab work on human cells has shown similar activity in the two clock genes observed in mice, suggesting that the find could be highly relevant to humans.
“The major circadian clock regulation mechanisms are conserved from mice to humans, including the two genes we studied,” says Lin. “Therefore we believe our research might point to physiological or clinical relevance in humans.”
Ataxin-2 has been found to be associated with multiple neurodegenerative diseases. For example, Ataxin-2 antisense oligonucleotides (ASO), which target RNA and modulate protein expression, have been suggested as a therapy for amyotrophic lateral sclerosis (ALS), a rare neurological disease that affects motor neurons. Considering the Ataxin-2 condensates oscillate along the circadian cycle, the results raise questions about when it is effective to administer such treatments, which should be the basis of further research, says Lin. “The timing of diagnosis and therapeutic interventions should be carefully considered in future,” she points out.
Reference
Zhuang, Y., Li, Z., Xiong, S., Sun, C., Li, B. et al. Circadian clocks are modulated by compartmentalized oscillating translation Cell 186 (15), P3245-3260.E23P3245 (2023)) doi: 10.1016/j.cell.2023.05.045
Editor: Guo Lili